Introduction
Assuring compliance of intermediate and final quality attributes in a continuous pharmaceutical manufacturing campaign is of utmost importance. Companies strive to produce medications of superior efficacy, safety and elegance, and to provide assurance to the physician, the pharmacist and the consumer that a given product performs uniformly and is fit for purpose. Drugs must be marketed as safe and use therapeutically active formulations whose performance is consistent and predictable. Incoming raw materials and production processes like stirring, pumping, and filling of pharmaceuticals have to be controlled by performing regular viscosity checks with a viscometer. The end product also needs to be tested to ensure proper storage stability or application. Parameters such as the viscosity of medicines have to be adapted to match customers’ expectations so that customer satisfaction is ensured. Process control of pharma manufacturing can reduce costs significantly by improving yield, reducing raw material variability and increasing delivery reliability.

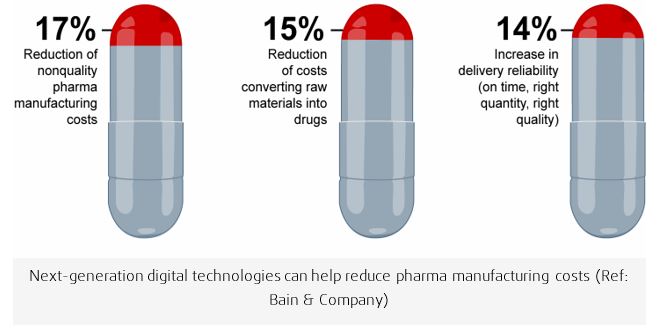
Preclinical submission contain relevant physical criteria reflecting the in vitro performance of a new drug. Results of tests concerning the disintegration time and dissolution rate of tablets, the weight variation of solid dosage forms, the viscosity of liquid and semiliquid preparations should be ascertained to illustrate that adequate control for ensuring uniform drug potency from batch to batch is maintained at all stages of production. As pharma executives grapple with rising complexity, costs and regulation, more of them are looking to Industry 4.0 manufacturing as a solution. Smart autonomous factories managed with data and machine learning will lower pharma manufacturing costs and enable application of corrective actions that might be required in real-time improve quality and reduce capacity constraints. Bain & Company reports that pharma executives expect smart connected factories to produce total savings of 20% or more, while improving quality and making deliveries more reliable. Specifically, they forecast a 17% reduction of costs related to poor quality, a 15% decline in the cost of converting raw materials into drugs and a 14% increase in delivery reliability.
Regulatory changes, the proliferation of generic drugs and more personalized medicine put pharma profits at risk and make a strong case for digital manufacturing. Smaller, more frequent drug launches increase production complexity and costs. The emergence of personalized medicine with drugs tailored to individual patients also means more complex processes. And widespread use of generic drugs is pushing prices down. For drug companies to remain competitive, it is crucial for them to have an agile, high performing manufacturing process and adopt IIoT and industry 4.0.
Process control: viscosity and density monitoring and control in pharma manufacturing
Despite the increased process knowledge and measurement techniques, control of the intermediate and final quality attributes in the pharmaceutical industry is still mainly based on a fixed recipe approach, where parameters are optimized once for a theoretical steady-state output, combined with acceptance sampling strategies. In reality, the appearance of changes in raw material properties, equipment status with respect to physical wear and varying ambient conditions contribute to disturbances which vary in time and demand for continuous corrective actions during production. Fluctuations in material throughput related to market demand can also be seen as a disturbance which needs to be compensated for to assure product quality. The traditional manufacturing approach which generally adopts automatic regulatory process control in combination with manual supervision, does not compensate in real-time for such critical quality attribute variations. To tackle these challenges, inclusion of automated supervisory process control in the manufacturing process becomes essential to automatically assure that critical quality attributes are consistently in agreement with the acceptance criteria in real-time.
Pharmaceutical types directly affected by manufacturing process control where viscosity plays a crucial role can be roughly divided into three main groups:
- Pharmaceutical solutions:These are homogeneous mixtures which do not show sedimentation of particles. Solutions consist of a soluble material which is dissolved in a liquid/solvent and looks clear. Solutions can be further divided into aqueous solutions like douches, mouthwash, and nasal wash, non-aqueous solutions like elixirs, spirits, and collodions, and sweet or viscid solutions like cough syrup and honey.
- Pharmaceutical emulsions:These are biphasic systems consisting of two immiscible liquids. They are thermodynamically unstable and must be stabilized by the addition of an emulsifying agent. Examples are preparations for the treatment of skin conditions and for skin care. An advantage of emulsions compared to solutions is that they can deliver water-insoluble drugs via oral administrations.
- Pharmaceutical suspensions: These are heterogeneous mixtures containing solid particles (>1000 nm) that settle down. A good suspension must be stable during storage, should settle slowly, and re-disperse readily upon gentle shaking of the container. Drugs that have very low solubility are usually formulated as suspensions. A suspension may prolong the action of a drug by preventing rapid degradation in the presence of water, improve chemical stability of certain drugs (e.g. penicillin G), and mask an unpleasant taste of a drug.
Viscosity Applications - Pharmaceuticals Industry Quality Control
| Challenge | Solution | Benefits |
|---|---|---|
| The moisturizing effect of the eye drops only lasts for a few seconds. | Analyse and precisely quantify the viscosity values of the methylcellulose solution in the eye drops at different temperatures, simulating real-life conditions (shear thinning effects during eye blinking and body temperature). | Simulate the consistency of the eye drops by determining the viscosity values at different shear rates and temperatures. Knowing these values, you can then adapt the portion of methylcellulose solution within the eye drops. |
| The cough syrup does not stay on the spoon and flows through the digestive tract too fast (so the surface of the throat is not covered). | Determine single-point viscosity for quick quality checks or make multi-point analyses at a constant temperature with an increasing speed. | Ensure the soothing effect and right viscosity of the cough syrup by using a single-point viscosity check or by studying the flow- or temperature-dependent behaviour. For a more in-depth analysis of the flow behaviour of the cough syrup, the mathematical model shear thinning index can be used. |
| The microemulsion separates into phases in heat (35 °C). | Analyse the temperature-dependent flow behaviour at a constant speed/shear rate. | Simulate the product’s behaviour during storage at different temperatures and improve the formula accordingly. |
| Clinical nourishments cannot be swallowed easily. | Determine single-point viscosity for quick quality checks or perform multi-point analyses at a constant temperature with increasing speed/shear rate. | By analysing the viscosity at low and high shear rates the viscosity of clinical nourishments at rest and during swallowing can be determined. Adding thickening agents to a low-viscosity fluid makes the fluid more viscous, which makes swallowing easier. |
| It is not possible to pump and squeeze ointments out of the packaging. During application on target body areas, good spread ability is not given. | Determine single-point viscosity for quick quality checks or perform multi-point analyses at a constant temperature with increasing speed/shear rate. | Ensure perfect pumpability and spread ability of ointments by using a single-point viscosity check or by studying the flow behaviour and the yield point. |
Why is viscosity management critical in pharmaceutical production?
The broad and significant factors which make viscosity management important in pharmaceutical production are:
- Product Quality: Medicines must comply with finished product specifications and any appropriate compendial requirements, and that can be ensured with viscosity quality control.
- Cost: Production with incorrect viscosity harms more than just quality. Poor viscosity management drives up usage of pigments and solvents, affecting the profit margins.
- Waste: Materials rejected due to poor quality can be reduced with proper viscosity management.
- Efficiency: Eliminating manual viscosity control frees operators’ time and enables them to focus on other tasks.
- Environment: Lowering the use of pigment and solvent is good for the environment.
- Compliance: Perhaps to a greater degree than other industries, pharmaceutical production demands the highest quality control. Correct composition and precisely controlled quality are non-negotiable when it comes to regulatory and traceability codes.
Simple viscosity checks with a viscometer ensures the correct consistency of the end product to meet customers’ expectations. Measurements of viscosity at different flow conditions give insight into the flow behaviour of pharmaceutical products. Yield point analysis helps to figure out the force required for e.g. pumping, filling, and application of pharmaceuticals. The viscosity, the flow behaviour, and the yield point can be controlled through tweaking the formulation of the product and addition of thickening agents.
To ensure consistent in-control manufacturing, the change in viscosity through-out the process stream is monitored in real time, making measurements from a baseline rather than simply measuring absolute values, and making viscosity adjustments by adjusting manufacturing processes (mixing, grinding, etc) and constituents to ensure consistency and accuracy of produced drugs.
Process Challenges
Existing laboratory viscometers are of little value in process environments because viscosity is directly affected by temperature, shear rate and other variables that are very different off-line from what they are in-line. Traditionally, operators have measured the viscosity of drug formulations using lab rotational viscometer or rheometers. The procedure is messy and time-consuming. Most often the batch is already finished before results arrive from the lab reducing the chances or corrections. Current viscosity measurement methods lead to inconsistent manufacturing, wastage of batches when they could have been corrected using in-line real-time monitoring. In addition, moving to continuous manufacturing requires in-line real-time process monitoring of formulation viscosity to ensure the process is within limits.
Vibrational instruments are used for in-line real-time monitoring of viscosity but they tend to be extremely bulky, slow reacting, are easily affected by external vibrations, require extensive maintenance and calibrations.
Rheonics' Solutions
Rheonics offers the best-in-class in-line viscometer, based on a balanced torsional resonator, for process control and optimisation in the processes of the pharmaceutical industry:
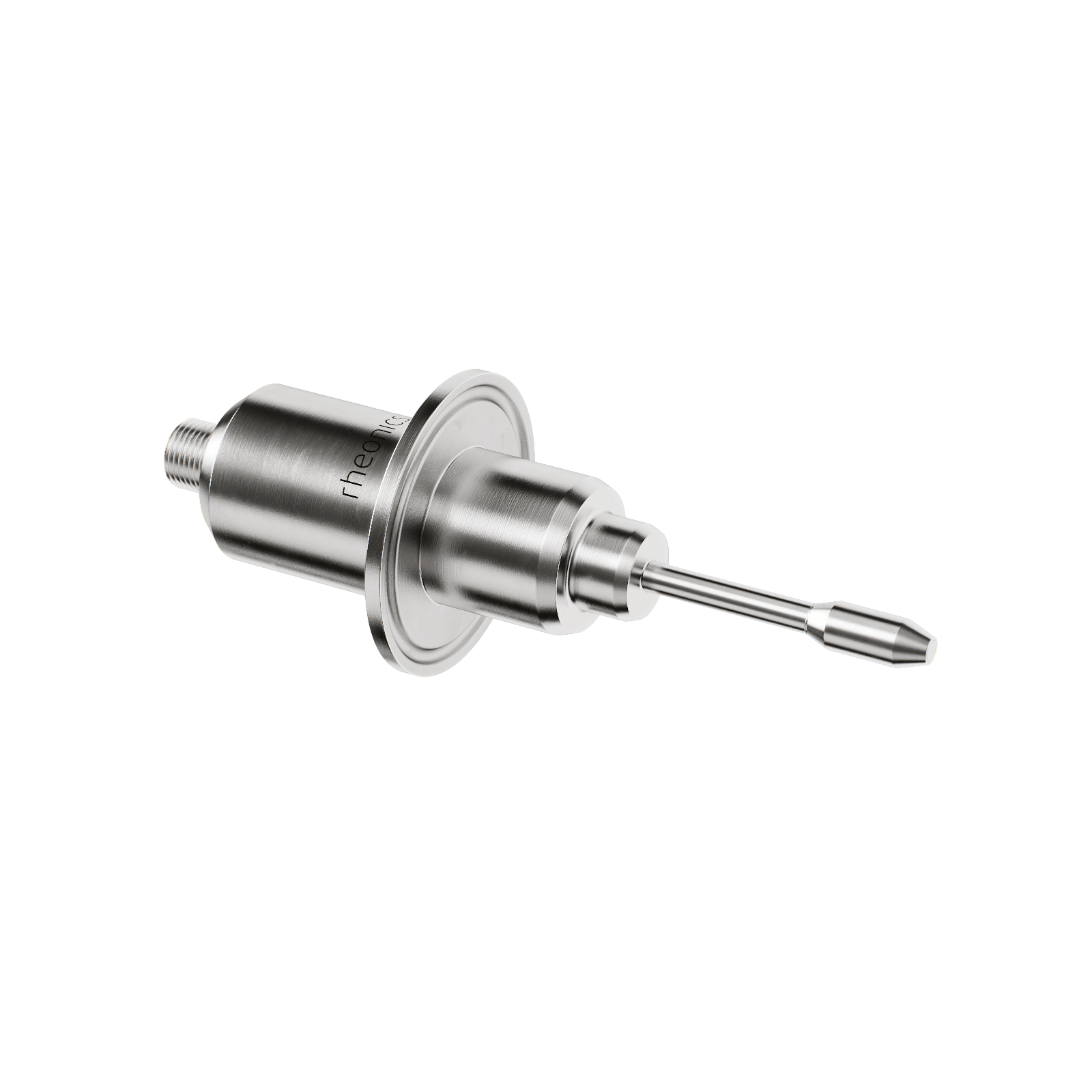
- In-line Viscosity measurements: Rheonics’ SRV is a wide range, in-line viscosity measurement device with in-built fluid temperature measurement and is capable of detecting viscosity changes within any process stream in real time.
- In-line Viscosity and Density measurements: Rheonics’ SRD is an in-line simultaneous density and viscosity measurement instrument with in-built fluid temperature measurement. If density measurement is important for your operations, SRD is the best sensor to cater to your needs, with operational capabilities similar to the SRV along with accurate density measurements.
Automated in-line viscosity measurement through SRV or an SRD eliminates the variations in sample taking and lab techniques which are used for viscosity measurement by the traditional methods. The sensor is located in-line so that it continuously measures the process fluid viscosity (and density in case of SRD). Manufacturing consistency is achieved through automation of the dosing system, mixers or pumps through a controller using continuous real-time viscosity measurements. Using an SRV in a pharma manufacturing line, product consistency is improved improving productivity, profit margins and environmental goals. Both the SRV and SRD have a compact form factor for simple OEM and retrofit installation. They require no maintenance or re-configurations. Both the sensors offer accurate, repeatable results no matter how or where they are mounted, without any need for special chambers, rubber seals or mechanical protection. SRV and SRD are available with Asceptic process connections like GEA Varinline and others. Using no consumables, SRV and SRD are extremely easy to operate.
Most pharma companies have adopted digital manufacturing tools slowly, worrying that their systems, data and people weren’t ready. Rheonics solutions are built to address the key challenges which operators in pharmaceuticals industry face and enable smooth integration of rheonics’ industrial solutions into your processes.
Once the manufacturing environment is established and process windows are adjusted to suit their proper purpose, there is usually little effort required to maintain the integrity of the manufacturing process with tight control on parameters with Rheonics viscosity control systems.
Rheonics' Advantage
Hygienic, sanitary design
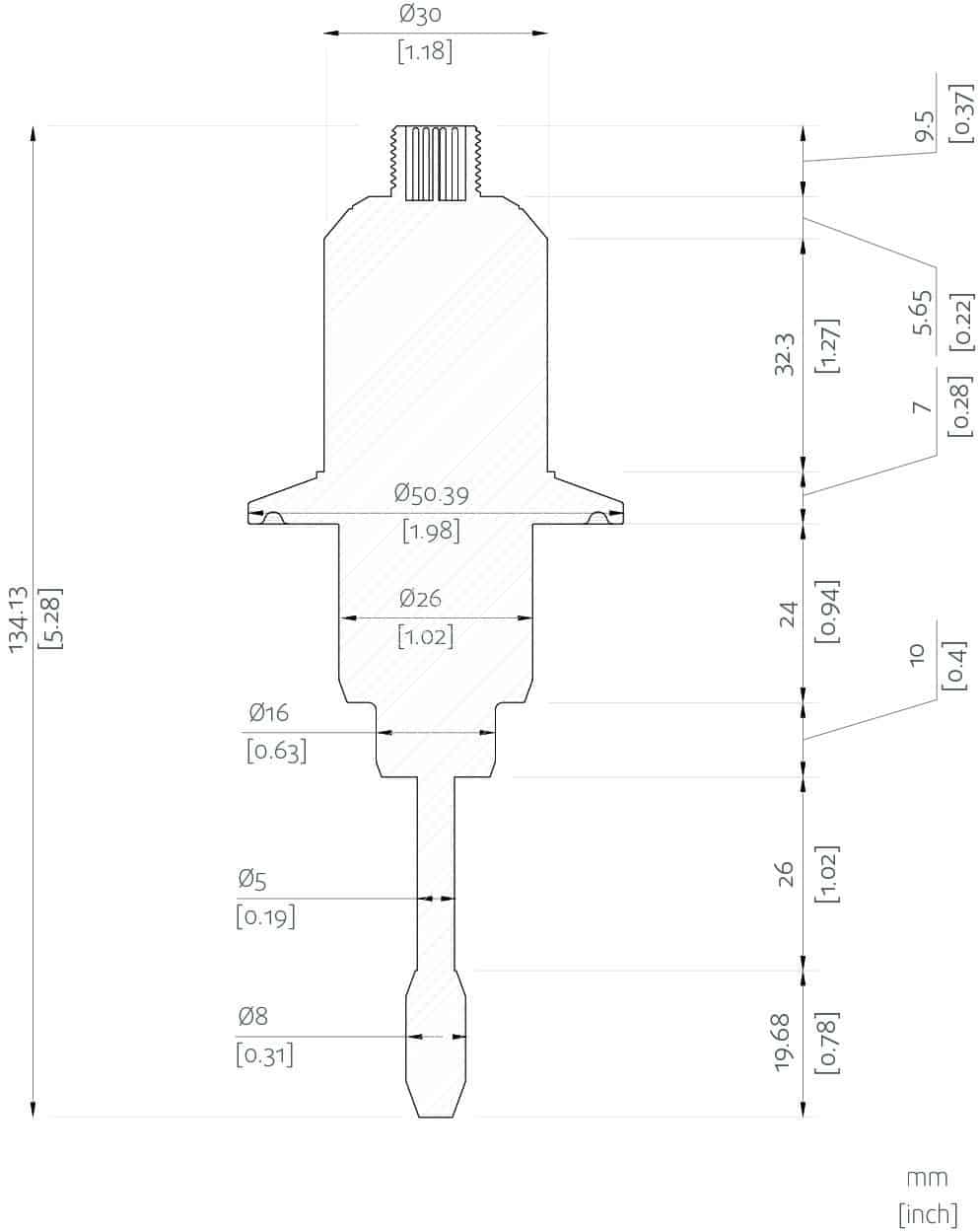
Rheonics SRV and SRD are available in tri-clamp and DIN 11851 connections besides custom process connections.
Compact form factor, no moving parts and require no maintenance
Rheonics’ SRV and SRD have a very small form factor for simple OEM and retrofit installation. They enable easy integration in any process stream. They are easy to clean and require no maintenance or re-configurations. They have a small footprint enabling Inline installation in ink lines, avoiding any additional space or adapter requirement on the process lines. Both sensors are available with Hygienic and Asceptic process connections.
High stability and insensitive to mounting conditions: Any configuration possible
Rheonics SRV and SRD use unique patented co-axial resonator, in which two ends of the sensors twist in opposite directions, cancelling out reaction torques on their mounting and hence making them completely insensitive to mounting conditions and process flow rates. These sensors can easily cope up with regular relocation. Sensor element sits directly in the fluid, with no special housing or protective cage required.

Instant accurate readouts on printing conditions – Complete system overview and predictive control
Rheonics’ software is powerful, intuitive and convenient to use. Real-time viscosity can be monitored on a computer. Multiple sensors are managed from a single dashboard spread across the factory floor. No effect of pressure pulsation from pumping on sensor operation or measurement accuracy. No effect of machine vibration.
Easy installation and no reconfigurations/recalibrations needed
Replace sensors without replacing or reprogramming electronics, drop-in replacements for both sensor and electronics without any firmware updates or calibration coefficient changes. Easy mounting. No chambers, O-ring seals or gaskets. Easily removed for cleaning or inspection. SRV available with asceptic flange and tri-clamp connection for easy mounting and dis-mounting.
SRV available with flange, DIN 11851 hygienic and tri-clamp connection for easy mounting and dis-mounting.
Advanced analytics for predictive maintenance
Using data from sensors to identify breakdown patterns—which part of a machine fails, the type of breakdown and when—this tool can predict problems in advance, giving production teams the chance to maintain machinery before it goes down. That early warning reduces production losses and helps prevent expensive repairs. And optimizing the frequency of maintenance also lowers its cost. Managers reviewing the system from end-to-end can rapidly spot problems and performance gaps and use data to identify the root causes.
Low power consumption
24V DC power supply with less than 0.1 A current draw during normal operation (less than 3W)
Fast response time and temperature compensated viscosity
Ultra-fast and robust electronics, combined with comprehensive computational models, make Rheonics devices one of the fastest and most accurate in the industry. SRV and SRD give real-time, accurate viscosity (and density for SRD) measurements every second and are not affected by flow rate variations!
Wide operational capabilities
Rheonics’ instruments are built to make measurements in the most challenging conditions. SRV has the widest operational range in the market for inline process viscometer:
- Pressure range up to 5000 psi and higher
- Temperature range from -40 up to 300°C
- Viscosity range: 0.5 cP up to 50,000+ cP
SRD: Single instrument, triple function – Viscosity, Temperature and Density
Rheonics’ SRD is a unique product that replaces three different instruments for viscosity, density and temperature measurements. It eliminates the difficulty of co-locating three different instruments and delivers extremely accurate and repeatable measurements in harshest of conditions.
Achieve the right production quality, cut down costs and enhance productivity
Integrate an SRV/SRD in the process line and ensure consistency throughout the manufacturing process. SRV (and SRD) constantly monitors and controls viscosity (and density in case of SRD) and prevents overuse of expensive pigments and solvents. Optimise the production process with an SRV and experience lower reject rates, lower waste, fewer customer complaints, fewer machine shut downs and improve material cost savings. And at the end of it all, it contributes to a better bottom line and a better environment!
Clean in place (CIP)
SRV (and SRD) monitors the cleanup of the process lines by monitoring the viscosity (and density) of the solvent during the cleaning phase. Any small residue is detected by the sensor, enabling the operator to decide when the line is clean for purpose. Alternatively, SRV (and SRD) provides information to the automated cleaning system to ensure full and repeatable cleaning between runs, thus ensuring full compliance in terms of sanitary standards of medicine manufacturing facilities.
Superior sensor design and technology
Sophisticated, patented 3rd generation electronics drive these sensors and evaluate their response. SRV and SRD are available with pharma industry standard process connections like asceptic GEA Varinline and Hygienic Tri-clamp allowing operators to replace an existing temperature sensor in their process line with SRV/SRD giving highly valuable and actionable process fluid information like viscosity besides an accurate measurement of temperature using an in-build Pt1000 (DIN EN 60751 Class AA, A, B available).
Environment friendly
Reduce the use of VOC (volatile organic compounds) in your process reducing the energy required to recover it or disposal costs. Manufacture smart while saving costs, ensuring high quality and protecting the environment.
Electronics built to fit your needs
Available in both an explosion-proof transmitter housing and a small-form factor DIN rail mount, the sensor electronics enables easy integration into process pipelines and inside equipment cabinets of machines.


Easy to integrate
Multiple Analog and digital communication methods implemented in the sensor electronics makes connecting to industrial PLC and control systems straightforward and simple.
ATEX & IECEx Compliance
Rheonics offers intrinsically safe sensors certified by ATEX and IECEx for use in hazardous environments. These sensors comply with the essential health and safety requirements relating to the design and construction of equipment and protective systems intended for use in potentially explosive atmospheres.
The intrinsically safe and explosion proof certifications held by Rheonics also allows for customization of an existing sensor, allowing our customers to avoid the time and costs associated with identifying and testing an alternative. Custom sensors can be provided for applications that require one unit up to thousands of units; with lead-times of weeks versus months.


Implementation
Directly install the sensor to your process stream to do real time viscosity and density measurements. No by-pass line is required: the sensor can be immersed in-line. Flow rate and vibrations do not affect the measurement stability and accuracy. Optimize coating performance by providing repeated, consecutive, and consistent tests on the fluid.
Rheonics Instrument Selection
Rheonics designs, manufactures and markets innovative fluid sensing and monitoring systems. Precision built in Switzerland, Rheonics’ in-line viscometers and density meters have the sensitivity demanded by the application and the reliability needed to survive in a harsh operating environment. Stable results – even under adverse flow conditions. No effect of pressure drop or flow rate. It is equally well suited to quality control measurements in the laboratory. No need to change any component or parameter to measure across full range.
Suggested product(s) for the Application
- Wide viscosity range – monitor the complete process
- Repeatable measurements in both Newtonian and non-Newtonian fluids, single phase and multi-phase fluids
- Hermetically sealed, all stainless steel 316L wetted parts
- Built in fluid temperature measurement
- Compact form-factor for simple installation in existing process lines
- Easy to clean, no maintenance or re-configurations needed
- Single instrument for process density, viscosity and temperature measurement
- Repeatable measurements in both newtonian and non-newtonian fluids, single phase and multi-phase fluids
- All metal (316L Stainless Steel) construction
- Built in fluid temperature measurement
- Compact form-factor for simple installation in existing pipes
- Easy to clean, no maintenance or re-configurations needed