- Innovate vaccine production and release, to address the global shortage of almost all vaccines
- Increase production capacity and shorten cycle times, especially in epidemic or pandemic setting
- Reliably detect reaction end points in bio reactors during production and use measurement data for conducting studies on product and process scalability
- Minimize costs and unpredictability of development and production
Introduction
Vaccine development has been supported by significant insights into bioprocess and analytical technologies. Such technologies have allowed vaccine manufacturers to achieve consistently high product purity and quality at a lower cost. Within the bioprocess industry, vaccine development and manufacture remain important and challenging due to the rapid growth of the vaccine market globally.
Due to its rapid growth, the global vaccine market has attracted new players. The World Health Organization (WHO) reports that, between 2000 and 2013, the vaccine market grew from 4 billion US dollars (USD) to 24 billion USD. It is predicted that by 2028, the vaccine market will be worth about 100 billion USD, growing at a compound annual growth rate (CAGR) of 11.02%. There are more than 120 new products in development, 60 of which are important to developing countries.
Vaccines are a booming market for the biopharmaceutical industry. Over the past few years, we have seen their status change within the industry as the number of mergers and acquisitions increases. New business models are emerging for vaccines, and they are generating considerable interest.
Complexities and Challenges
Vaccines are large, complex often hybrid biological molecules. They are produced through multiple steps of production and formulation for which the end product (vaccine or combination vaccine) is often a combination of many component products (antigens or vaccines). Although vaccines are biological products derived from living organisms, they are more complex than many traditional therapeutics, both in terms of their components and the technologies required to produce them. They are typically administered to healthy individuals (prophylaxis), whereas other therapies are given to persons with medical conditions. They also are more difficult to develop and manufacture than many other biologics and thus are more difficult to make in “generic” form. So, vaccine products are more likely to retain their commercial value.
Vaccines are manufactured using a wide range of cell substrates (e.g., mammalian, insect, microbial, and fungal cell lines). New antigens also typically require novel cell substrates. The list includes a diversity of vaccine products, including live attenuated vaccines, inactivated or detoxified vaccines, subunit vaccines, polysaccharides, virus-like particles, and protein complexes. Each vaccine type has its own degree of complexity and range of biochemical and biological properties.
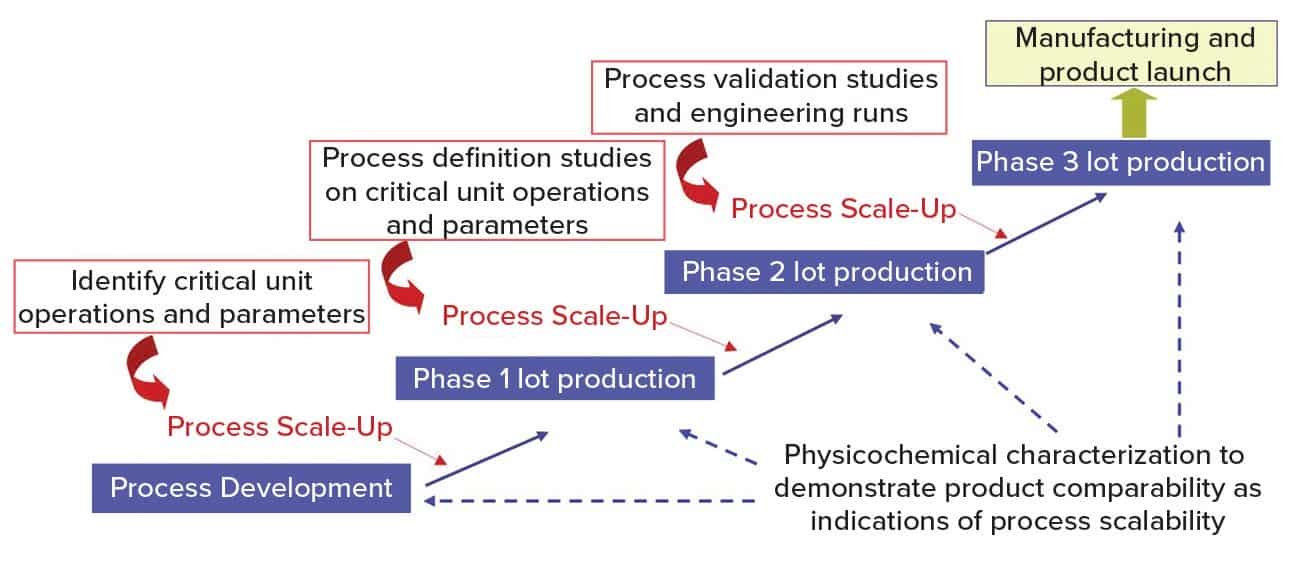
Figure 1 shows a general approach for vaccine process development for drug substance. Process development starts at a laboratory scale for identification of unit operations and parameters, followed by step-wise scale-up (usually in a 20-L fermentation or cell culture) for production of good manufacturing practice (GMP) materials for phase 1 clinical studies. Process definition studies are performed on critical unit operations and parameters using a design of experiments (DoE) before scaling up to 200 L (normally for phase 2). Before phase 3 (~2,000-L scale), process validation and engineering runs are required. During process development and scale-up, scientists perform biophysical, chemical, and biological characterization to acquire product and process knowledge to support and demonstrate product comparability and process scalability.
In addition to the above complexities of development, vaccine manufacturers face a high-cost and high-risk business environment, competition with other major vaccine manufacturers, increasing compliance and safety expectations, and highly sophisticated technology-driven platforms. Developing and licensing a vaccine product typically takes 12–14 years (Figure 2). Total costs can exceed $1 billion per new development, and the overall success rate from early phase development to license was <10% during 2000–2010. A 2016 study showed a ~20% success rate from phase 1 to license.

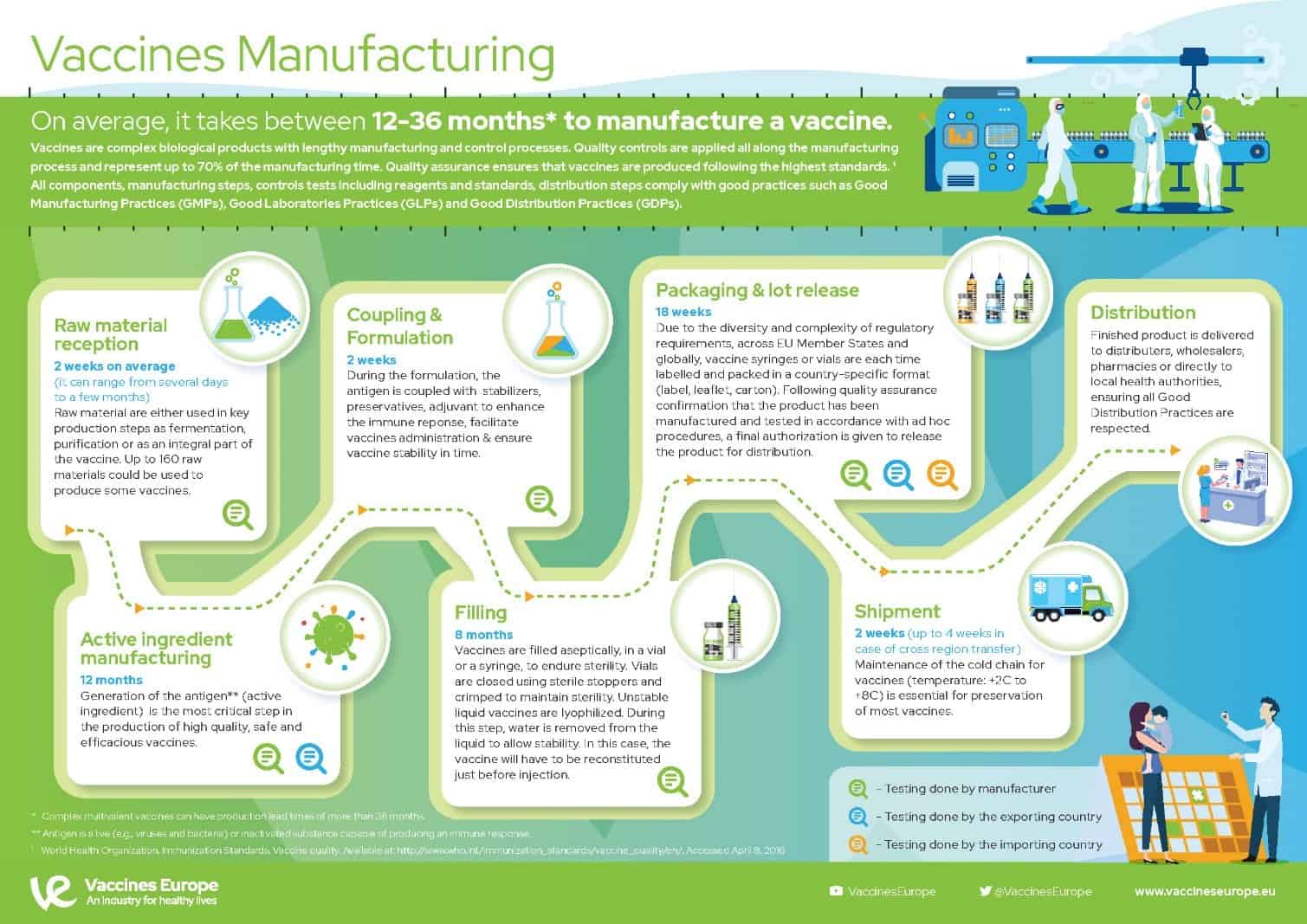
Making a vaccine at a glance
Source: AstraZeneca | Innovating Production and Manufacture to meet the Challenge of COVID-19
These are the crucial manufacturing processes needed to create a vaccine on an unprecedented scale:
- CMC – A commercial manufacturing process has been developed from an initial small-scale process before rapidly scaling to increase productivity yields, all the time ensuring purity of the final product. This consistent process is employed by each manufacturing facility we are collaborating with.
- Virus seed stock & host cell bank – These starting materials are used by manufacturers around the world to initiate vaccine production.
- Drug substance – Host cells are grown in a series of bioreactors of increasing scale and infected by the virus seed to produce a final vaccine molecule. A series of filtration and chromatography steps are taken to harvest and purify the vaccine.
- Drug product – The drug substance is combined with buffers to achieve a final formulation and then filled into multi-dose vials, which are labelled and packaged.
- Testing and quality control – Extensive testing is conducted on every batch throughout the manufacturing process. Quality control measures are employed at all stages of production to ensure consistency and quality.
Key Trends
Changing market: The vaccine industry is challenged to develop high-quality products at decreased cost and within shortened timelines. The need to reach the market first highlights the importance for fast process development strategies and techniques. Such pressures have driven the vaccine industry to embrace innovative technologies. In return, shortening process development times will accelerate overall vaccine product development timelines and rapidly deliver safe and high-quality products to a global market.
CIP Requirements: Some vaccine manufacturers face additional difficulties. These include the need to work with small batch sizes and varied product portfolios. Pandemic outbreaks that require rapid responses from vaccine developers and use of highly potent ingredients place large demands on cleaning processes.
Accelerating product development with single-use technology: Application of single-use technologies provide opportunities to reduce fixed costs, amount of equipment, and cleaning validation while increasing facility and process flexibility and accelerating process development time. Disposables play a key role in addressing the industrial challenges associated with developing high quality processes while driving down operational costs.
The net result is an overall reduction in development time and manufacturing costs. Facility turnover is easier and faster with this than with the previous system, and validation of fixed assets such as stainless-steel fermenters, tanks, and centrifuges is no longer necessary.
A closed system demonstrates the benefit of applying disposable technology to accelerate process development. Implementation of such technologies is anticipated to reduce process development time, trim manufacturing costs, and increase process and facility flexibility, thus facilitating expansion of manufacturing activities and increasing process development efficiency.

Vaccine Classification
There are many approaches to vaccine development, but vaccines can be broadly classified by how the antigen(s), the active component(s) that generate a specific immune response against the disease-causing organism, are prepared. Vaccines may be viral (live or inactivated), viral vector, subunit (protein or polysaccharide) or nucleic acid (DNA or RNA). Combination vaccines may include inactivated, protein-based and/or protein-conjugated polysaccharide vaccine components. Other ingredients in vaccines vary depending on the manufacturing process and the nature of the antigen(s).
- Live attenuated vaccines
- Inactivated or dead vaccines
- Subunit vaccines
- Protein vaccines
- Pure polysaccharide vaccines
- Nucleic acid-based vaccines
Viscosity measurement device: Use cases in vaccine manufacturing
Despite the increased process knowledge and measurement techniques, control of the intermediate and final quality attributes in the pharmaceutical industry is still mainly based on a fixed recipe approach, where parameters are optimized once for a theoretical steady-state output, combined with acceptance sampling strategies. In reality, the appearance of changes in raw material properties, equipment status with respect to physical wear and varying ambient conditions contribute to disturbances which vary in time and demand for continuous corrective actions during production. Fluctuations in material throughput related to market demand can also be seen as a disturbance which needs to be compensated for to assure product quality. The traditional manufacturing approach which generally adopts automatic regulatory process control in combination with manual supervision, does not compensate in real-time for such critical quality attribute variations. To tackle these challenges, inclusion of automated supervisory process control in the manufacturing process becomes essential to automatically assure that critical quality attributes are consistently in agreement with the acceptance criteria in real-time. This becomes particularly important in vaccine manufacturing processes.
Control of polysaccharide – Reaction end point monitoring and data logging
A vaccine manufacturer should demonstrate consistency of the degree of modification of the polysaccharide, either by an assay of each batch of the polysaccharide or by validation of the manufacturing process. Depending on the conjugation chemistry used, consistency in degree of polysaccharide activation may be determined as part of process validation or reflected by characteristics of vaccine lots shown to have adequate safety and immunogenicity in clinical trials.
The degree of size reduction of the polysaccharide will depend upon the manufacturing process. The average size distribution (degree of polymerization) of the modified polysaccharide should be determined by a suitable method and shown to be consistent. The molecular size distribution should be specified for each serotype, with appropriate limits for consistency, as the size may affect the reproducibility of the conjugation process.
Viscosity sensors measure the progress of a chemical reaction that takes place during acid fragmentation or polysaccharide synthesis during vaccine production. Polysaccharide chain length influences viscosity. Viscosity reduction should be continuously monitored with reaction time, preventing the reaction from continuing beyond the viscosity set point. The use of a viscometer embedded inline with the ability to perform reliable, accurate and continuous measurements of viscosity in order to detect reaction end points and to record and store measurement data can streamline production processes and improve quality control.
Vaccine adjuvant characterization and quality control with viscosity/density measurements
Adjuvants (immune potentiators or immunomodulators) have been used for decades to improve the immune response to vaccine antigens. The incorporation of adjuvants into vaccine formulations is aimed at enhancing, accelerating and prolonging the specific immune response towards the desired response to vaccine antigens.
A vaccine adjuvant is a component that potentiates the immune responses to an antigen and/or modulates it towards the desired immune responses. An active ingredient of a combined vaccine that has an adjuvant effect on other active ingredients of the vaccine is excluded from the scope of this Guideline. Also excluded are carriers for haptens, antigens (e.g., CRM197, meningococcal OMP, tetanus toxoid and diphtheria toxoid that are used to conjugate polysaccharides) and excipients such as HSA. More than one adjuvant may be present in the final vaccine product.
The results an assessment of a number of parameters used to characterise the adjuvant should be described. Critical parameters should be identified and described. Such parameters are likely to be part of the routine testing of batches of the adjuvant. Other parameters will also be analysed to characterise the adjuvant and some of these may also form part of routine testing. The parameters which define an adjuvant will depend on the nature of the adjuvant and may include, but will not necessarily be limited to:
- chemical composition (qualitative and quantitative)
- physical characteristics (e.g., visual appearance, density, viscosity, pH, size and size distribution, surface charge)
- biochemical characteristics
- purity (e.g., endotoxin content, bioburden, manufacturing residuals)
Viscosity/density measurements can support scientists who are required to perform biophysical, chemical, and biological characterizations on vaccine products and processes to demonstrate and support product comparability and process scalability.
What is the significance of viscosity quality control in vaccine production?
Viscosity management has broad and significant implications for vaccine production for the following reasons:
- Quality: Inline process viscosity control for reaction end-point detection can make sure vaccine specifications and compendial requirements are met. In manufacturing, quality control is necessary for ensuring lot-to-lot consistency and releasing the product to the market, which can be enabled by analyzing viscosity/density measures.
- Cost: The consequences of improper viscosity extend beyond product quality. Material usage increases as a result of poor viscosity control, negatively impacting profit margins.
- Waste: Material rejections due to low quality can be minimized when viscosity is managed effectively in continuous production.
- Efficiency: Eliminating manual viscosity control with laboratory measurement devices frees operators’ time and enables them to focus on other tasks.
- Environment: Lowering the use of materials and solvents will affect the environment positively.
- Compliance: Perhaps to a greater degree than other industries, pharmaceutical production demands the highest quality control. Correct composition and precisely controlled quality are non-negotiable when it comes to regulatory and traceability codes.
- Supports transition to a continuous production process and Pharma 4.0: Viscosity sensor data provide access to data for digitalizing pharmaceutical manufacturing, bringing transparency and adaptability. The system further improves decision-making speed; is able to cope with smaller batch sizes and a broader product portfolio – improving quality control in real-time through real-time quality monitoring.
Viscosity measurements can determine the concentration of dissolved solids in a solution. Monitoring viscosity improves the understanding of process conditions, reduces drug development time, increases production capacity and stability, ensures product quality, and can help demonstrate compliance with regulations. Pharmaceutical manufacturers are required to demonstrate process validation from drug discovery to production, and this can be attained by viscosity measurements. Viscosity measurements are important in characterizing physicochemical properties (density, viscosity, surface tension, osmolality, glass transition temperature) of mRNA-loaded LNP intermediate and the finished product solutions at different temperatures.
Process challenges
Upscaling production of vaccinations is difficult due to mixing problems of the components. Real-time viscosity measurements can help in determining ideal processing and mixing parameters by analyzing rheological properties and aiding the design of upscale from small lab-scale to large industrial processes by knowing the viscous properties. Additionally, it helps precisely control the quality during manufacturing. To ensure consistent in-control manufacturing, the change in viscosity through-out the process stream is monitored in real time, making measurements from a baseline rather than simply measuring absolute values, and making viscosity adjustments by adjusting manufacturing processes (mixing, grinding, etc) and constituents to ensure consistency and accuracy of produced drugs.
Sucrose density measurements are particularly useful during the influenza virus purification process. With these reliable measurements, influenza vaccines can be developed as quickly and as safely as possible without compromising quality.
Existing laboratory viscometers are of little value in process environments because viscosity is directly affected by temperature, shear rate and other variables that are very different off-line from what they are in-line. Traditionally, operators have measured the viscosity of formulations using lab rotational viscometer or rheometers. The procedure is messy and time-consuming. Most often the batch is already finished before results arrive from the lab reducing the chances or corrections. Current traditional viscosity measurement methods lead to inconsistent manufacturing, wastage of batches when they could have been corrected using in-line real-time monitoring. In addition, moving to continuous manufacturing requires in-line real-time process monitoring of formulation viscosity to ensure the process is within limits.
Vibrational instruments are used for in-line real-time monitoring of viscosity but they tend to be extremely bulky, slow reacting, are easily affected by external vibrations, require extensive maintenance and calibrations. Some of the challenges for sensors in vaccine production environment are high heat and humidity, regular cleaning requirements and environmental compensations for measurements.
Rheonics’ Solutions
Rheonics offers the best-in-class in-line viscometer, based on a balanced torsional resonator, for process control and optimisation in the processes of the pharmaceutical industry:
- In-line Viscosity measurements: Rheonics’ SRV is a is a wide range, in-line viscosity measurement device with inbuilt fluid temperature measurement and is capable of detecting viscosity changes within any process stream in real time. It can be used in bio reactors and vessels to reliably detect reaction end-points and automatically stop the reaction by integrating with any factory automation system.
- In-line Viscosity and Density measurements: Rheonics’ SRD is an in-line simultaneous density and viscosity measurement instrument with inbuilt fluid temperature measurement. If density measurement is important for your operations, SRD is the best sensor to cater to your needs, with operational capabilities similar to the SRV along with accurate density measurements.
Automated in-line viscosity measurement through SRV or an SRD eliminates the variations in sample taking and lab techniques which are used for viscosity measurement by the traditional methods. The sensor is located in-line so that it continuously measures the process fluid viscosity (and density in case of SRD). Manufacturing consistency is achieved through automation of the dosing system, mixers or pumps through a controller using continuous real-time viscosity measurements. Using an SRV in a pharma manufacturing line, product consistency is improved improving productivity, profit margins and environmental goals. Both the SRV and SRD have a compact form factor for simple OEM and retrofit installation. They require no maintenance or re-configurations. Both the sensors offer accurate, repeatable results no matter how or where they are mounted, without any need for special chambers, rubber seals or mechanical protection. SRV and SRD are available with asceptic process connections like GEA Varinline and others. Using no consumables, SRV and SRD are extremely easy to operate.
Most pharma companies have adopted digital manufacturing tools slowly, worrying that their systems, data and people weren’t ready. Rheonics solutions are built to address the key challenges which operators in pharmaceuticals industry face and enable smooth integration of rheonics’ industrial solutions into your processes.
Once the manufacturing environment is established and process windows are adjusted to suit their proper purpose, there is usually little effort required to maintain the integrity of the manufacturing process with tight control on parameters with Rheonics viscosity control systems.
Rheonics' Advantage
Rheonics viscometers and density meters offer the pharmaceutical industry many specifically designed technological advantages. These include:
- Pharma grade wetted materials: Stainless steel AISI316L
- Electro-polished wetted materials to secure surface roughness of Ra<0.4μm/15μ inch
- No animal derived ingredients (ADI) is used
- Equipment scalability and qualification
Compact form factor, no moving parts and require no maintenance
Rheonics’ SRV and SRD have a very small form factor for simple OEM and retrofit installation. They enable easy integration in any process stream. They are easy to clean and require no maintenance or re-configurations. They have a small footprint enabling inline installation in circulation lines, avoiding any additional space or adapter requirement on the process lines. Both sensors are available with Hygienic and Asceptic process connections.
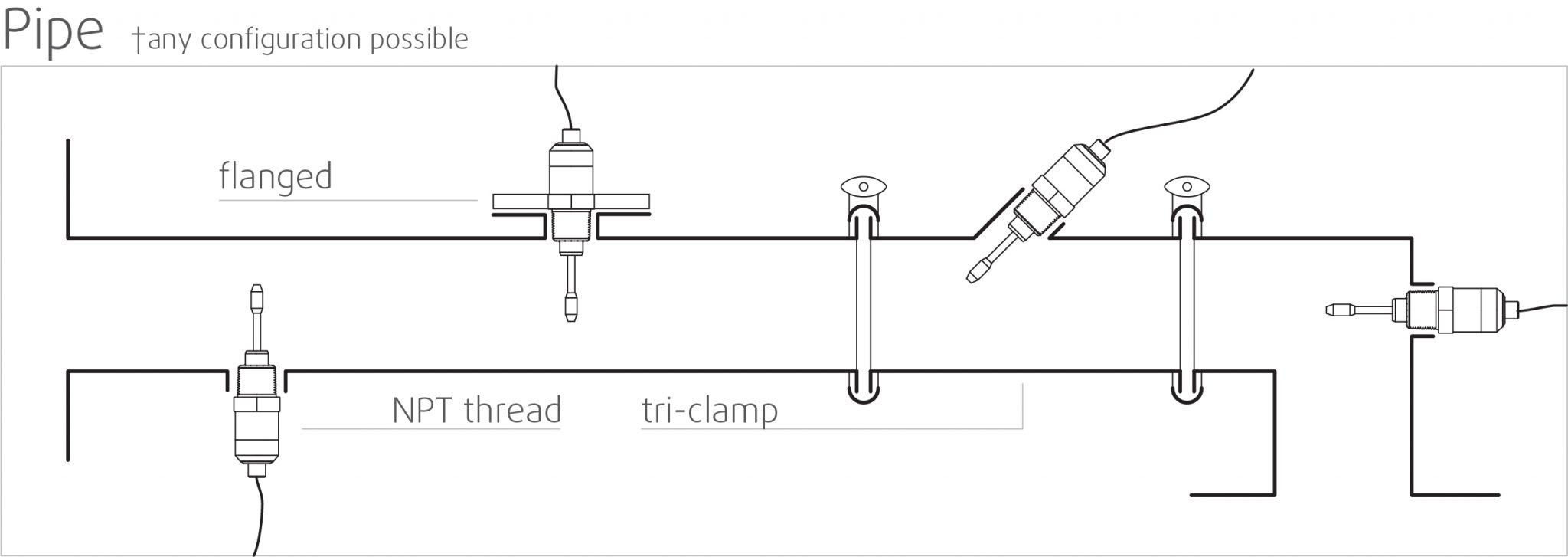
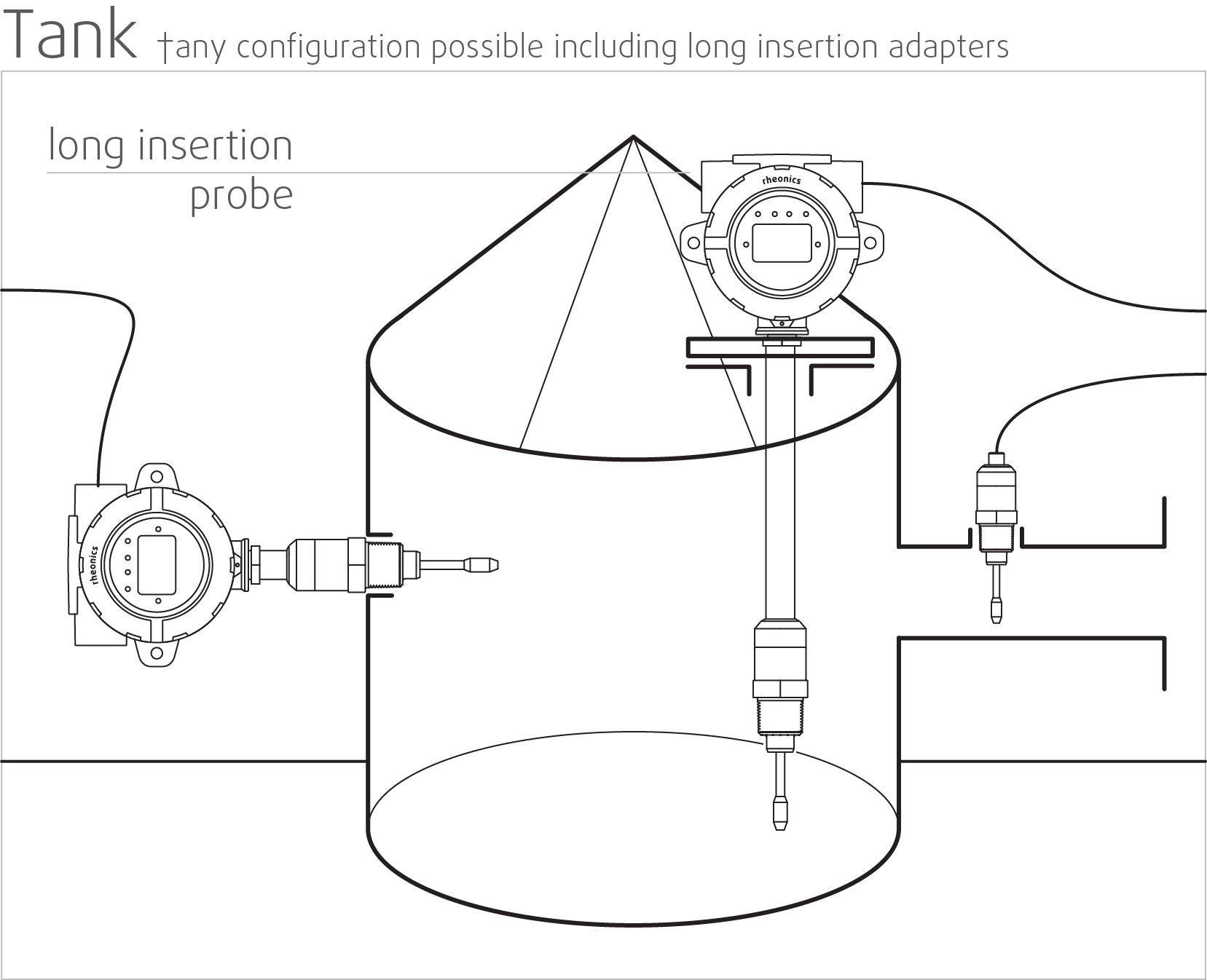
High stability and insensitive to mounting conditions: Any configuration possible
Rheonics SRV and SRD use unique patented co-axial resonator, in which two ends of the sensors twist in opposite directions, cancelling out reaction torques on their mounting and hence making them completely insensitive to mounting conditions and flow rates. Sensor element sits directly in the fluid, with no special housing or protective cage requirements.
Instant accurate readouts on production quality – Complete system overview and predictive control
Rheonics’ software is powerful, intuitive and convenient to use. Real-time viscosity can be monitored on a computer. Multiple sensors are managed from a single dashboard spread across the factory floor. No effect of pressure pulsation from pumping on sensor operation or measurement accuracy. No effect of machine vibration.
Easy installation and no reconfigurations/recalibrations needed – least maintenance/down-times
Replace sensors without replacing or reprogramming electronics, drop-in replacements for both sensor and electronics without any firmware updates or calibration coefficient changes. Easy mounting. No chambers, O-ring seals or gaskets. Easily removed for cleaning or inspection. SRV available with asceptic flange and tri-clamp connection for easy mounting and dis-mounting.
Advanced analytics for predictive maintenance
Using data from sensors to identify breakdown patterns—which part of a machine fails, the type of breakdown and when—this tool can predict problems in advance, giving production teams the chance to maintain machinery before it goes down. That early warning reduces production losses and helps prevent expensive repairs. And optimizing the frequency of maintenance also lowers its cost. Managers reviewing the system from end-to-end can rapidly spot problems and performance gaps and use data to identify the root causes.
Low power consumption
24V DC power supply with less than 0.1 A current draw during normal operation.
Fast response time and temperature compensated viscosity
Ultra-fast and robust electronics, combined with comprehensive computational models, make Rheonics devices one of the fastest, versatile and most accurate in the industry. SRV and SRD give real-time, accurate viscosity (and density for SRD) measurements every second and are not affected by flow rate variations!
Wide operational capabilities
Rheonics’ instruments are built to make measurements in the most challenging conditions.
- Pressure range up to 5000 psi
- Temperature range from -40 up to 200°C
SRV has the widest operational range in the market for inline process viscometer:
- Viscosity range: 0.5 cP up to 50,000 cP
SRD: Single instrument, triple function – Viscosity, Temperature and Density
Rheonics’ SRD is a unique product that replaces three different instruments for viscosity, density and temperature measurements. It eliminates the difficulty of co-locating three different instruments and delivers extremely accurate and repeatable measurements in harshest of conditions.
- Viscosity range: 0.5 cP up to 3,000 cP
- Density range: 0 up to 4 g/cc (0 to 4000 kg/m3)
Achieve accurate lubricant quality information through direct measurements, cut down costs and enhance productivity
Integrate an SRV/SRD in the process line to schedule lubricant change intervals optimally and achieve significant cost savings. Compared to the indirect approach of using algorithms to predict the real state, lubricant viscosity measurements would yield a true physical picture of the lubrication allowing the detection of possible approaching bearing/engine failures or abnormal states. And at the end of it all, it contributes to a better bottom line and a better environment!
Clean in place (CIP)
SRV (and SRD) are self-cleaning sensors – using the in-line fluid to clean the sensor while it is taking measurements reduces unscheduled maintenance. Any small residue is detected by the sensor, enabling the operator to decide when the line is clean for purpose. Alternatively, these sensors provide information to the automated cleaning system to ensure full and repeatable cleaning between production runs.
Superior sensor design and technology
Sophisticated, patented electronics is the brain of these sensors. SRV and SRD are available with industry standard process connections like ¾” NPT, DIN 11851, Flange and Tri-clamp allowing operators to replace an existing temperature sensor in their process line with SRV/SRD giving highly valuable and actionable process fluid information like viscosity besides an accurate measurement of temperature using an in-build Pt1000 (DIN EN 60751 Class AA, A, B available).
Electronics built to fit your needs
Available in both a transmitter housing and a small-form factor DIN rail mount, the sensor electronics enables easy integration into process lines and inside equipment cabinets of machines.
Easy to integrate
Multiple Analog and digital communication methods implemented in the sensor electronics makes connecting to industrial PLC and control systems straightforward and simple.
Analog and Digital Communication Options
Optional Digital Communication Options
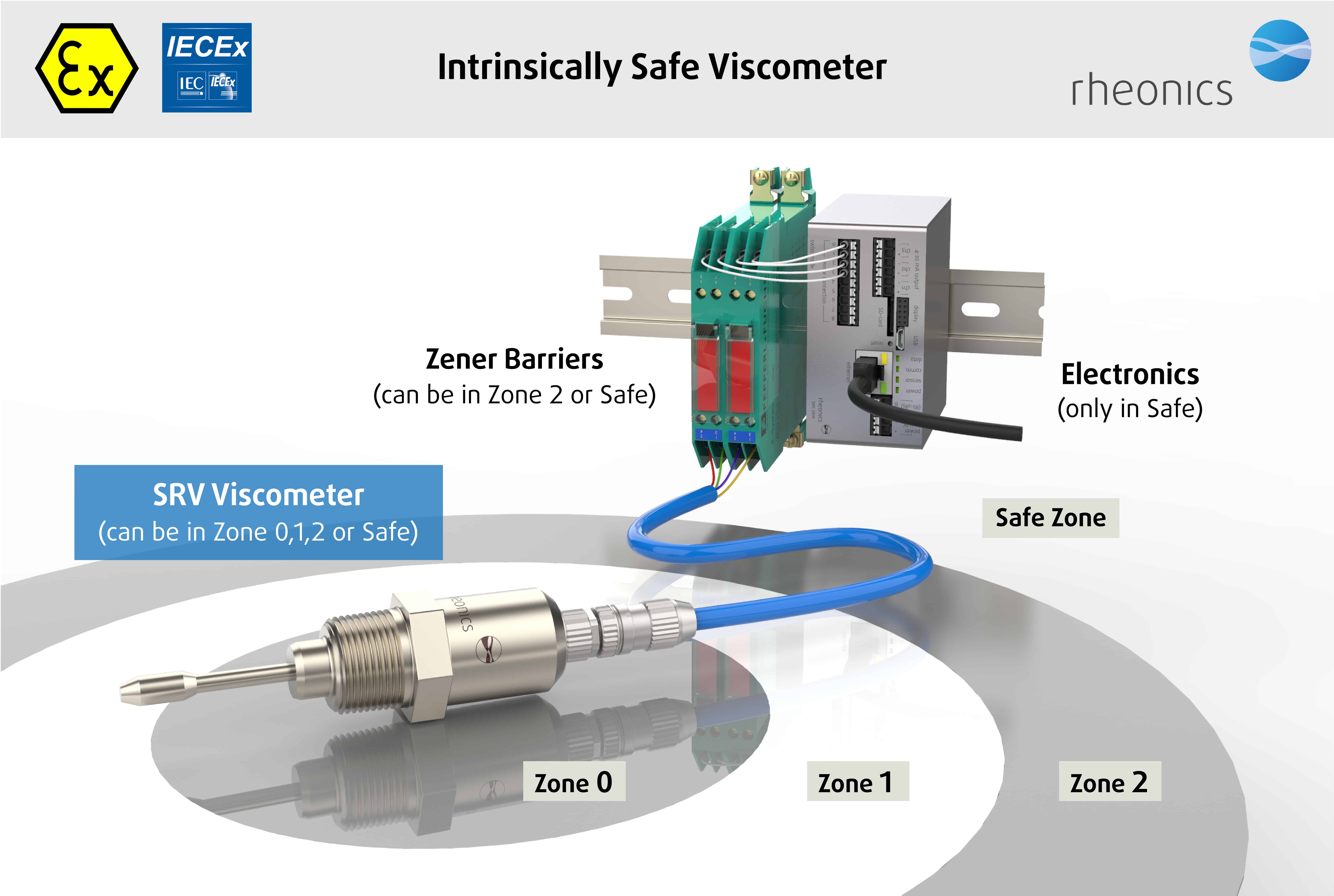
ATEX & IECEx Compliance
Rheonics offers intrinsically safe sensors certified by ATEX and IECEx for use in hazardous environments. These sensors comply with the essential health and safety requirements relating to the design and construction of equipment and protective systems intended for use in potentially explosive atmospheres.
The intrinsically safe and explosion proof certifications held by Rheonics also allows for customization of an existing sensor, allowing our customers to avoid the time and costs associated with identifying and testing an alternative. Custom sensors can be provided for applications that require one unit up to thousands of units; with lead-times of weeks versus months.


Implementation
Directly install the sensor in your process stream to do real-time viscosity and density measurements. No bypass line is required: the sensor can be immersed in-line; flow rate and vibrations do not affect the measurement stability and accuracy. Optimize mixing performance by providing repeated, consecutive, and consistent tests on the fluid.
In-line Quality control locations
- In bioreactors for reaction monitoring
- In the connecting pipes between various processing containers
Instruments/Sensors
SRV Viscometer OR an SRD for additional density
Rheonics Instrument Selection
Rheonics designs, manufactures and markets innovative fluid sensing and monitoring systems. Precision built in Switzerland, Rheonics’ in-line viscometers and density meters have the sensitivity demanded by the application and the reliability needed to survive in a harsh operating environment. Stable results – even under adverse flow conditions. No effect of pressure drop or flow rate. It is equally well suited to quality control measurements in the laboratory. No need to change any component or parameter to measure across full range.
Suggested product(s) for the Application
- Wide viscosity range – monitor the complete process
- Repeatable measurements in both Newtonian and non-Newtonian fluids, single phase and multi-phase fluids
- Hermetically sealed, all stainless steel 316L wetted parts
- Built in fluid temperature measurement
- Compact form-factor for simple installation in existing process lines
- Easy to clean, no maintenance or re-configurations needed
- Single instrument for process density, viscosity and temperature measurement
- Repeatable measurements in both newtonian and non-newtonian fluids, single phase and multi-phase fluids
- All metal (316L Stainless Steel) construction
- Built in fluid temperature measurement
- Compact form-factor for simple installation in existing pipes
- Easy to clean, no maintenance or re-configurations needed