Introduction
Product marking has come under increased scrutiny in the pharmaceutical industry due to pressure from three factors: regulations, aesthetics, and cost. In order to prevent dispensing errors and medication errors in tablets handled at medical sites, tablets with excellent visual discrimination are required. Apart from the regulatory requirements, which identification scheme manufacturers of SODFs (Solid Oral Dosage Forms) select depends on their goals for branding, marketing, artwork, and dosing strategy. The identification technology options include debossing, printing (traditional printing, continuous inkjet printing and thermal inkjet printing) and ultraviolet laser marking – the method chosen by the manufacturer depends on content, format, and complexity of the identifiers desired/required to achieve business needs. For pharmaceutical manufacturers, digital printing holds the promise of better brand protection and another layer of deterrence against counterfeiting. On-dose markings can show trademarks, product names, dose strength and manufacturer details.

Advances in identification technology coupled with smart phones and other personal electronics equip Internet of Things to pharmaceutical tablets and capsules. Printing data matrix codes onto tablets can ensure to authenticate single tablets and capsules being administered to individual patients/subjects. Drug interaction warnings can be effectively issued through printing on tablets. Patients could simply scan tablets and capsules to verify identity and then get data from an online database about potentially dangerous combinations.
Application
Pharmaceutical companies strive to differentiate their products from competing products. A product’s “trade dress”- its physical characteristics, such as shape, size, colour and printing – is a unique combination of features that qualifies as intellectual property protected by law in many countries.
Tablet debossing can cater to the basic requirements of tablet identification, however there are limits to the size and type of debossing that a tablet press punch can provide. Debossing techniques make the tablets susceptible to picking and sticking which leads to poor tablet quality and incomplete or missing identification features on the tablets, potentially leading to rejection of such tablets. UV laser marking provides indelible marking on metals, plastics, ceramics, composites, and semi-conductors with ease and precision. However, inkjet printing still dominates in specific applications where colours are needed for logo or safety requirements (i.e. Yellow or Red). Lasers can only mark on a grey-scale, so colouring is impossible. Even if lasers achieve colours, repeatability is extremely difficult. Ink‐Jet printing provides full RGB or CMYK colour swatches, and is best used for markings that require colouring like warning or hazard labels – of extreme relevance in pharma-print applications.
Printing onto finished tablets and capsules with ink enables manufacturers to include detailed logos or symbols and to print in multiple colours, increasing the number of possible identification of schemes. It’s a mature technology and in use for more than 60 years. The basic approach is to transfer ink from an engraved pattern onto rubber roll or pad and then onto the tablet/capsule. There are several techniques in traditional printing – rotogravure and tampo printing, both offering similar capabilities and differing mainly in their throughput.
Inkjet printing is a recent method, which gained acceptance in the pharmaceutical industry. It offers greatest versatility in terms of printing schemes and multiple colours, complex logos, and machine-readable codes. With continuous inkjet printing, the print format and data sequencing can be easily changed using software. Inkjet printing offers an alternate to debossing uncoated tablets, some of which are too soft for debossing (E.g. – Orally Disintegrating Tablets ODTs).
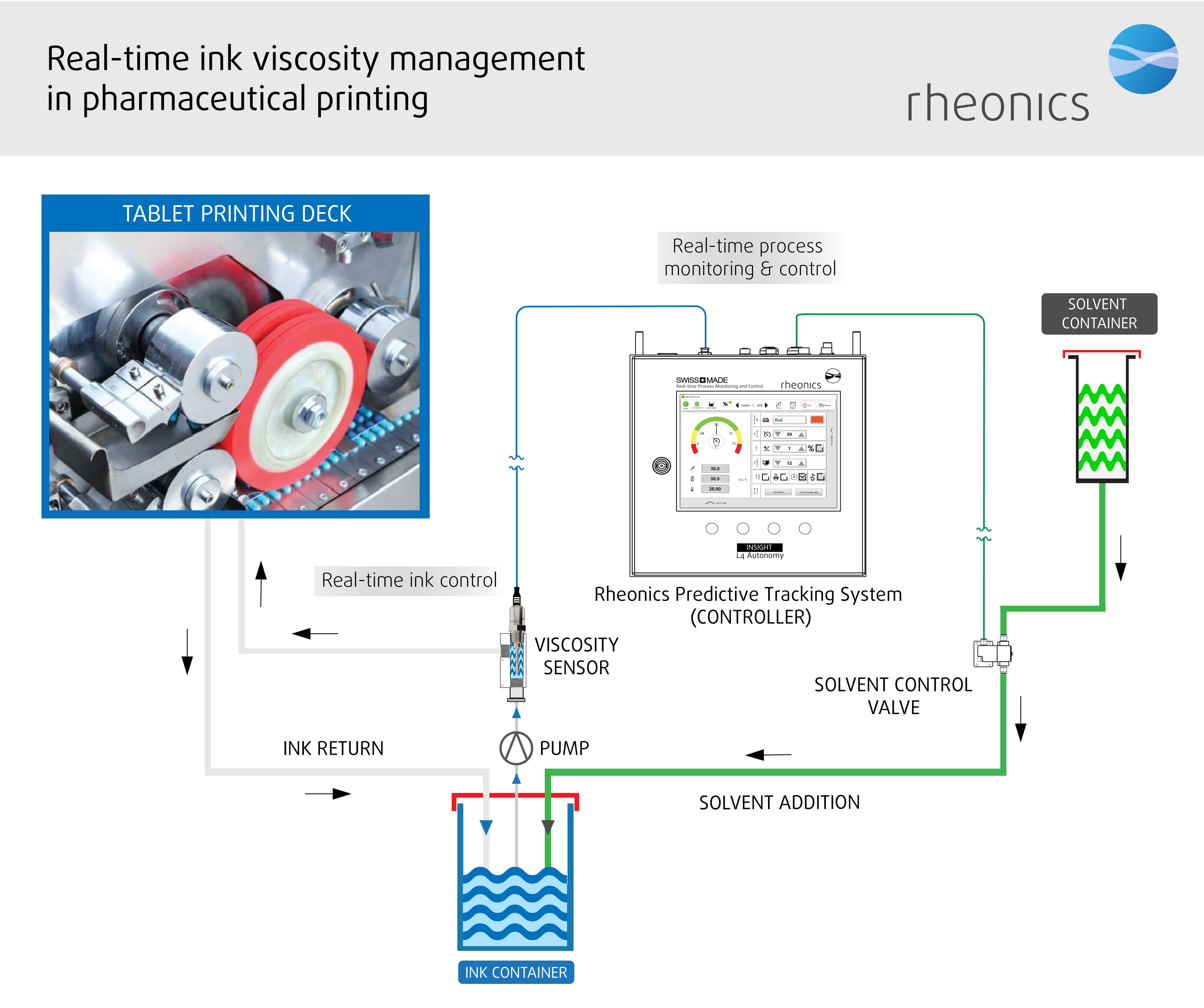
With continuous inkjet printing, a recirculating stream of ink is supplied to a nozzle that applies it to the tablet or capsule. Any ink that is not used flows bank into the reservoir. Ink droplets are produced through vibration of print head using a piezoelectric crystal and are deflected by energised electrodes to impinge on the product’s surface. The process is extremely fast to produce precise, sharp imprints with good resolution. The bulk of ink used in the printers recirculates continuously, which leads to solvent loss through evaporation. The evaporative rate of the thinners used can have an effect on print quality and run ability. To ensure consistent print quality, a control loop based on ink viscosity is used to add makeup solvent. The controls enable to orchestrate the operation of all the components to achieve the desired results.
In order to optimise efficiency and quality, it is absolutely necessary to compensate for solvent loss by adding small amount of thinner from time to time, during the operation, to keep the viscosity optimum for use through adjustments as conditions dictate.
Why is viscosity management critical in pharma-printing process?
The broad and significant factors which make viscosity management critical in pharmaceutical printing are:
- Print Quality: Tablets must comply with finished product specifications and any appropriate compendial requirements, and that can be ensured. Variation in viscosity causes significant change in both solvent and water-based ink properties affecting printability, fade resistance and drying.
- Reduce printing errors: Viscosity control can help alleviate the frequency of miscues – sticking and picking, print weak (thin line), print broad (fuzzy and washed out), print missing (incomplete) and smudged (spotty marked printed product).
- Colour: Colour consistency and colour density are highly critical to the right print quality. Controlling ink viscosity is the key to colour consistency because that is the factor subjected to the highest variability. The percent solids of fluid is the characteristic of the ink that gives it colour. Ink viscosity is an indicator to the percent solids of the fluid.
- Cost: Printing with incorrect viscosity harms more than just quality. Poor viscosity management drives up usage of pigments and solvents, affecting the profit margins.
- Waste: Materials rejected due to poor quality can be reduced with proper viscosity management.
- Efficiency: Eliminating manual viscosity control frees operators’ time and enables them to focus on other tasks.
- Environment: Lowering the use of pigment and solvent is good for the environment.
- Compliance: On-dose identification helps in product differentiation and enhances product safety. Perhaps to a greater degree than other industries, pharmaceutical printing demands the highest quality printing. Legibility and contrast are non-negotiable when it comes to regulatory and traceability codes.
Once the printing environment is established and inks are adjusted to suit their proper purpose, there is usually little effort required to maintain the integrity of the printing inks. To ensure consistent high-quality printing, the change in ink viscosity through-out the process stream is monitored in real time, making measurements from a baseline rather than simply measuring absolute values, and making viscosity adjustments by adjusting solvents and temperature to keep it within specified limits.
Process Challenges
Existing laboratory viscometers are of little value in process environments because viscosity is directly affected by temperature, shear rate and other variables that are very different off-line from what they are in-line. Traditionally, operators have measured the viscosity of printing ink using the efflux cup or Zahn cup. The procedure is messy and time-consuming, particularly if the ink needs to be filtered first. It is pretty inaccurate, inconsistent and non-repeatable even with an experienced operator.
Some companies use thermal management systems to keep point-of-application at a determined optimal temperature to achieve constant ink viscosity. But temperature is not the only factor affecting viscosity. Shear rate, flow conditions, pressure and other variables can affect viscosity changes as well. Temperature controlled systems also have long installation times and a large footprint.
Conventional vibrational viscometers are unbalanced, requiring large masses to avoid large influence of mounting forces.
Rheonics' Solutions
Automated in-line viscosity measurement and control is crucial to control the ink viscosity. Rheonics offers the following solutions, based on a balanced torsional resonator, for process control and optimisation in the printing process:
- In-line Viscosity measurements: Rheonics’ SRV is a is a wide range, in-line viscosity measurement device with inbuilt fluid temperature measurement and is capable of detecting viscosity changes within any process stream in real time.
- In-line Viscosity and Density measurements: Rheonics’ SRD is an in-line simultaneous density and viscosity measurement instrument with inbuilt fluid temperature measurement. If density measurement is important for your operations, SRD is the best sensor to cater to your needs, with operational capabilities similar to the SRV along with accurate density measurements.
Automated in-line viscosity measurement through SRV or an SRD eliminates the variations in sample taking and lab techniques which are used for viscosity measurement by the traditional methods. The sensor is located in-line so that it continuously measures the ink viscosity (and density in case of SRD). Printing consistency is achieved through automation of the dosing system through a controller using continuous real-time viscosity measurements. Using an SRV in a printing process line, ink transfer efficiency is improved improving productivity, profit margins and environmental goals. Both the sensors have a compact form factor for simple OEM and retrofit installation. They require no maintenance or re-configurations. Both the sensors offer accurate, repeatable results no matter how or where they are mounted, without any need for special chambers, rubber seals or mechanical protection. Using no consumables, SRV and SRD are extremely easy to operate.
Once the printing environment is established and inks are adjusted to suit their proper purpose, there is usually little effort required to maintain the integrity of the printing inks with tight control on parameters with Rheonics ink viscosity control systems.
Compact form factor, no moving parts and require no maintenance
Rheonics’ SRV and SRD have a very small form factor for simple OEM and retrofit installation. They enable easy integration in any process stream. They are easy to clean and require no maintenance or re-configurations. They have a small footprint enabling Inline installation in ink lines, avoiding any additional space or adapter requirement on the press and on ink carts.
High stability and insensitive to mounting conditions: Any configuration possible
Rheonics SRV and SRD use unique patented co-axial resonator, in which two ends of the sensors twist in opposite directions, cancelling out reaction torques on their mounting and hence making them completely insensitive to mounting conditions and ink flow rates. These sensors can easily cope up with regular relocation. Sensor element sits directly in the fluid, with no special housing or protective cage required.
Instant accurate readouts on printing conditions – Complete system overview and predictive control
Rheonics’ software is powerful, intuitive and convenient to use. Real-time ink viscosity can be monitored on a computer. Multiple sensors are managed from a single dashboard spread across the factory floor. No effect of pressure pulsation from pumping on sensor operation or measurement accuracy. No effect of printing press vibration.
Easy installation and no reconfigurations/recalibrations needed – least maintenance/down-times
Replace sensors without replacing or reprogramming electronics, drop-in replacements for both sensor and electronics without any firmware updates or calibration coefficient changes. Easy mounting. Screws into ¾” NPT thread in ink line fitting. No chambers, O-ring seals or gaskets. Easily removed for cleaning or inspection. SRV available with flange and tri-clamp connection for easy mounting and dis-mounting.
Low power consumption
24V DC power supply with less than 0.1 A current draw during normal operation
Fast response time and temperature compensated viscosity
Ultra-fast and robust electronics, combined with comprehensive computational models, make Rheonics devices one of the fastest and most accurate in the industry. SRV and SRD give real time, accurate viscosity (and density for SRD) measurements every second and are not affected by flow rate variations!
Wide operational capabilities
Rheonics’ instruments are built to make measurements in the most challenging conditions. SRV has the widest operational range in the market for inline process viscometer:
- Pressure range up to 5000 psi
- Temperature range from -40 up to 200°C
- Viscosity range: 0.5 cP up to 50,000 cP
SRD: Single instrument, triple function – Viscosity, Temperature and Density
Rheonics’ SRD is a unique product that replaces three different instruments for viscosity, density and temperature measurements. It eliminates the difficulty of co-locating three different instruments and delivers extremely accurate and repeatable measurements in harshest of conditions.
Achieve the right print quality, cut down costs and enhance productivity
Integrate an SRV/SRD in the process line and ensure colour consistency throughout the printing process. Achieve constant colours without worrying about colour variations. SRV (and SRD) constantly monitors and controls viscosity (and density in case of SRD) and prevents overuse of expensive pigments and solvents. Reliable and automatic ink supply ensures that presses run faster and saves operators’ time. Optimise the printing process with an SRV and experience lesser reject rates, lesser wastes, fewer customer complaints, fewer press shut downs and material cost savings. And at the end of it all, it contributes to a better bottom line and a better environment!
Clean in place (CIP)
SRV (and SRD) monitors the cleanup of the ink lines by monitoring the viscosity (and density) of the solvent during the cleaning phase. Any small residue is detected by the sensor, enabling the operator to decide when the line is clean for purpose. Alternatively, SRV (and SRD) provides information to the automated cleaning system to ensure full and repeatable cleaning between runs, thus ensuring full compliance in terms of sanitary standards of medicine manufacturing facilities.
Superior sensor design and technology
Sophisticated, patented 3rd generation electronics drive these sensors and evaluate their response. SRV and SRD are available with industry standard process connections like ¾” NPT and 1” Tri-clamp allowing operators to replace an existing temperature sensor in their process line with SRV/SRD giving highly valuable and actionable process fluid information like viscosity besides an accurate measurement of temperature using an in-build Pt1000 (DIN EN 60751 Class AA, A, B available).
Electronics built to fit your needs
Available in both an explosion-proof transmitter housing and a small-form factor DIN rail mount, the sensor electronics enables easy integration into process pipelines and inside equipment cabinets of machines.


Easy to integrate
Multiple Analog and digital communication methods implemented in the sensor electronics makes connecting to industrial PLC and control systems straightforward and simple.
Implementation
Directly install the sensor to your process stream to do real time viscosity and density measurements. No bypass line is required: the sensor can be immersed in-line, flow rate and vibrations do not affect the measurement stability and accuracy. Optimize decision making process by providing repeated, consecutive, and consistent tests on the fluid.
Rheonics Instrument Selection
Rheonics designs, manufactures and markets innovative fluid sensing and monitoring systems. Precision built in Switzerland, Rheonics’ in-line viscometers has the sensitivity demanded by the application and the reliability needed to survive in a harsh operating environment. Stable results – even under adverse flow conditions. No effect of pressure drop or flow rate. It is equally well suited to quality control measurements in the laboratory.
Suggested product(s) for the Application
• Wide viscosity range – monitor the complete process
• Repeatable measurements in both newtonian and non-newtonian fluids, single phase and multi-phase fluids
• Hermetically sealed, all Titanium Grade 5 wetted parts
• Built in fluid temperature measurement
• Compact form-factor for simple installation in existing process lines
• Hermetically sealed, all Titanium Grade 5 wetted parts
• Easy to clean, no maintenance or re-configurations needed
• Single instrument for process density, viscosity and temperature measurement
• Repeatable measurements in both newtonian and non-newtonian fluids, single phase and multi-phase fluids
• All metal (316L Stainless Steel) construction
• Built in fluid temperature measurement
• Compact form-factor for simple installation in existing pipes
• Easy to clean, no maintenance or re-configurations needed




