What are emulsions : examples, features, preparation & measurement
Introduction
Emulsions are dispersions of two immiscible liquids. They are of interest in many important practical applications in the food, cosmetic, oil production, agriculture, chemical, oil & gas, pharmaceuticals and several other process industries.

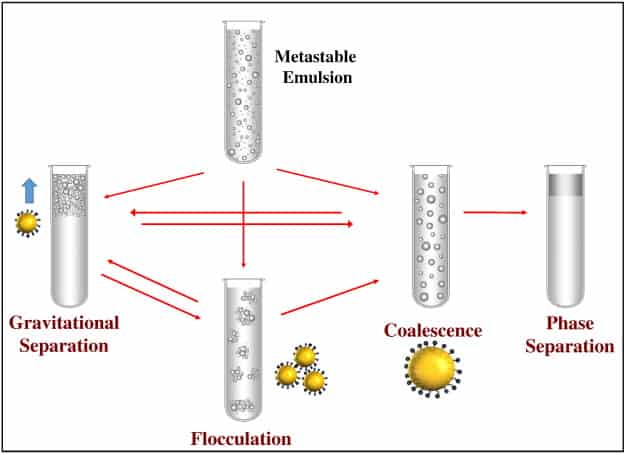
There are two types of emulsions: Oil-in-water (O/W) type, where oil forms the dispersed (droplets) phase and water forms the continuous phase; and water-in-oil (W/O) type, where water forms the dispersed (droplets) phase and oil forms the continuous phase. Without an effective interfacial stabilizer, emulsions are unstable systems and they readily separate out into oil and water phases as the mechanical agitation is stopped.
One of the most important of emulsions is their inherent instability. Though the dispersed drops are small, gravity exerts a measurable force on them and overtime they coalesce to form larger drops which tend to either settle to the bottom or rise to the top of the mixture. This process ultimately causes the internal and external phases to separate into the two original components. Depending on how the emulsion is formulated and the physical environment to which it is exposed, this separation may take minutes, months, or millennia.
Application areas & industries - Emulsions
- Milk is an emulsion of milk fat in an aqueous solution containing many different proteins, lactose and salts. In raw milk the fat is present in the form of milk fat globules, which are surrounded by a membrane. When this milk is homogenized in the factory, these globules are broken, and the fat is dispersed into smaller droplets, also stabilized by proteins.
- Margarine is an emulsion of water droplets in fat, stabilised by a packing of needle-like crystals of fat inside the continuous fat phase.
- Cream is a concentrated emulsion of milk fat in an aqueous phase; the concentration depends on the type of cream.
- Ice cream is a very complex product; amongst others it contains droplets of milk fat, but it also contains crystals of sugar, ice crystals and bubbles of air.
- Salad dressings are made by emulsifying vegetable oil in an aqueous mixture that contains vinegar. When made at home, this emulsion is rather unstable: the droplets coalesce relatively quickly, so one has to shake it before use. Commercial variants are usually stabilized by other components.
- Mayonnaise is a very concentrated emulsion of oil droplets in water, stabilised by proteins from egg yolk. The emulsion is so concentrated (70–80 vol.%) that the oil droplets are squeezed together. This squeezing together causes the nice consistency of mayonnaise.
- Egg yolk is an emulsion of egg fat (and cholesterol) in an aqueous solution, stabilised by a mixture of phospholipids.
- Food products. Salad dressings, gravies and other sauces, whipped dessert toppings, peanut butter, and ice cream are also examples of emulsions of various edible fats and oils. In addition to affecting the physical form of food products, emulsions impact taste because emulsified oils coat the tongue, imparting “mouth-feel.”
- Waterborne paints and coatings are usually emulsions of polymer-based binder particles. They are made by making an emulsion of droplets of monomers in water, after which the monomers are polymerized to form solid particles. When applied, the water and possibly other solvents evaporate, and the binder particles fuse to form a solid layer.
- Bitumen, a heavy fraction produced in the refining of petroleum, is usually too viscous to be applied directly. Therefore, bitumen is emulsified in water at high The resulting O/W emulsions have a much lower viscosity, and therefore are easier to apply. When applied (on the road or on a roof), the emulsion breaks, and the bitumen particles fuse into one layer.
- Medicines & Drugs. Starch/Gelatin Blend Microparticles are prepared by the water-in-oil emulsion solvent diffusion method. The in vitro drug release content significantly depends on the starch blend ratio and the crosslinker ratio. Starch/gelatin blend microparticles should be a useful controlled release delivery carrier for water-soluble drugs. In the pharmaceutical industry, emulsions are used to make medicines more palatable, to improve effectiveness by controlling dosage of active ingredients, delayed release drugs and to provide improved aesthetics for topical drugs such as ointments.
- Oils and hydrocarbons. Two third parts of the worldwide crude oils are produced in an emulsified way; these emulsions are mainly of the water-in-oil type due to the production processes.
- Insecticides and pesticides. In the agricultural industry, emulsions are used as delivery vehicles for insecticides, fungicides and pesticides and are applied usually by spraying through mechanical equipment.
- In cosmetics, emulsions are the delivery vehicle for many hair and skin conditioning agents. Anionic and non-ionic emulsions are used to deliver various oils and waxes which provide moisturization, smoothness and softness to hair and skin. Other examples are face creams, body lotions, shampoos, shower gels, toothpastes and fragrances.
- Lubricants, slurries, additives, machine oils, polymer emulsions, glues, starch solutions, mineral filler slurries, textile emulsions, submicron emulsions and silicone emulsions.
- Battery Materials – Water-based binders for batteries are developed by utilizing advanced polymer technologies, to enable the formation of negative electrodes in lithium-ion secondary batteries and nickel-hydrogen secondary batteries. Compared to conventional battery binders (PVDF), these binders have excellent binding properties, electrolyte resistance and cycle properties.
Key points and features of emulsions
- An emulsion is a type of colloid formed by combining two liquids that normally don’t mix.
- In an emulsion, one liquid contains a dispersion of the other liquid.
- The process of mixing liquids to form an emulsion is called emulsification.
- Even though the liquids that form them may be clear, emulsions appear cloudy or colored because light is scattered by the suspended particles in the mixture.
- Safety and environmental friendliness ensured by using water as a solvent
- Viscosity can be easily adjusted
- Suitability for gluing and coating applications derived from emulsion’s property of forming a film when dried
- Excellent miscibility with pigments, solvents, additives, etc
Preparing emulsions
An emulsion is prepared by shaking strongly the mixture of the two liquids or by passing the mixture through a colloid mill known as the homogenizer. The emulsions thus prepared from the pure liquids are usually not stable and the two liquids separate out on standing. To get a stable emulsion, small quantities of certain other substances are added during preparation. The substances thus added to stabilize the emulsions are called emulsifiers or emulsifying agents. The substances commonly used as emulsifying agents are soaps of various kinds, long chain sulphonic acids or lyophilic colloids like proteins, gum, and agar.
Emulsions may be prepared by several methods, depending upon the nature of the components and the equipment. On a small scale, as in the laboratory or pharmacy, emulsions may be prepared using a dry Wedgwood or porcelain mortar and pestle or a mechanical blender or mixer. On a large scale, large mixing tanks may be used to form the emulsion through the action of a high-speed impeller.

Viscosity of emulsions
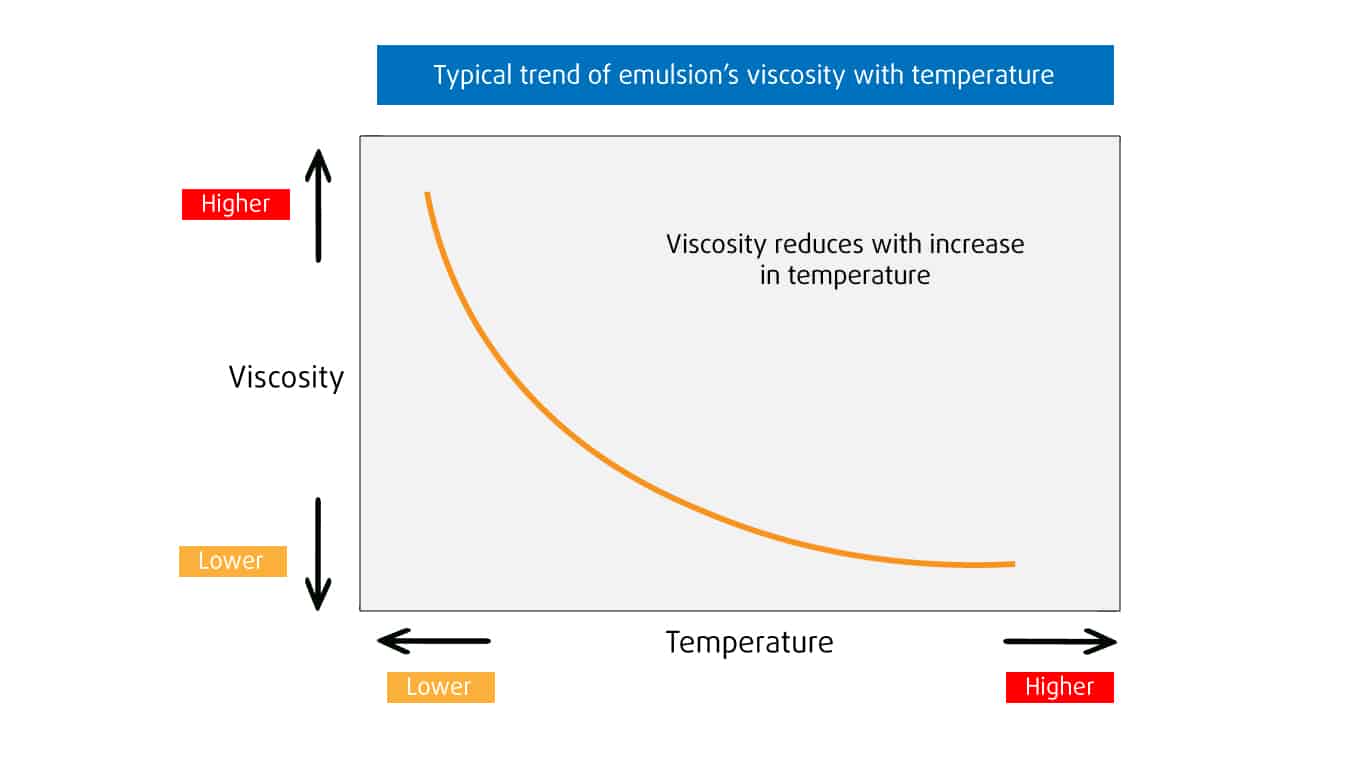
- The viscosity of emulsions varies depending on the temperature.
- As the temperature decreases, the viscosity increases.
- By adding water, the viscosity can be adjusted to a lower viscosity
- To increase the viscosity, emulsion thickeners may be used
Viscosity measurements of emulsions for Quality control
Viscosity measurement is an extremely useful technique for quality control.
- During homogenization, many emulsions will undergo a substantial viscosity increase as the droplet size is reduced. The amount of this increase will, therefore, be a good indicator of emulsion quality.
- Concentration of the emulsion bears strong correlation with the emulsion viscosity, hence the viscosity information can be effectively used to predict concentrations with pre-defined or user-defined models.
- During the mixing process, characterizing the viscosity – inline can be useful in determining the emulsion stability, desired end point of the mixing/blending system and to ensure continuity. If need arises, adjustments to stirring intensity, rotational speed, equipment may be made depending on the measured viscosity data and its interpretations.
- Emulsions are complex systems with a wide range of applications and commercial uses. Accurate characterization of emulsions with the viscosity data is critical to ensure emulsion stability and performance.
Viscosity measurement obtained with an inline viscometer can provide an excellent QC benchmark and ensure QA/QC of the process and end-product. Please read our application note.
Rheonics density meters and viscosity meters are available as probes and flow-through systems for installation in blending skids, storage tanks, loading terminal, process lines and in transport vessels. All Rheonics products are designed to withstand harshest process environments, high temperature, high level of shock, vibrations, abrasives & chemicals.
Unique advantages with the SRV/SRD
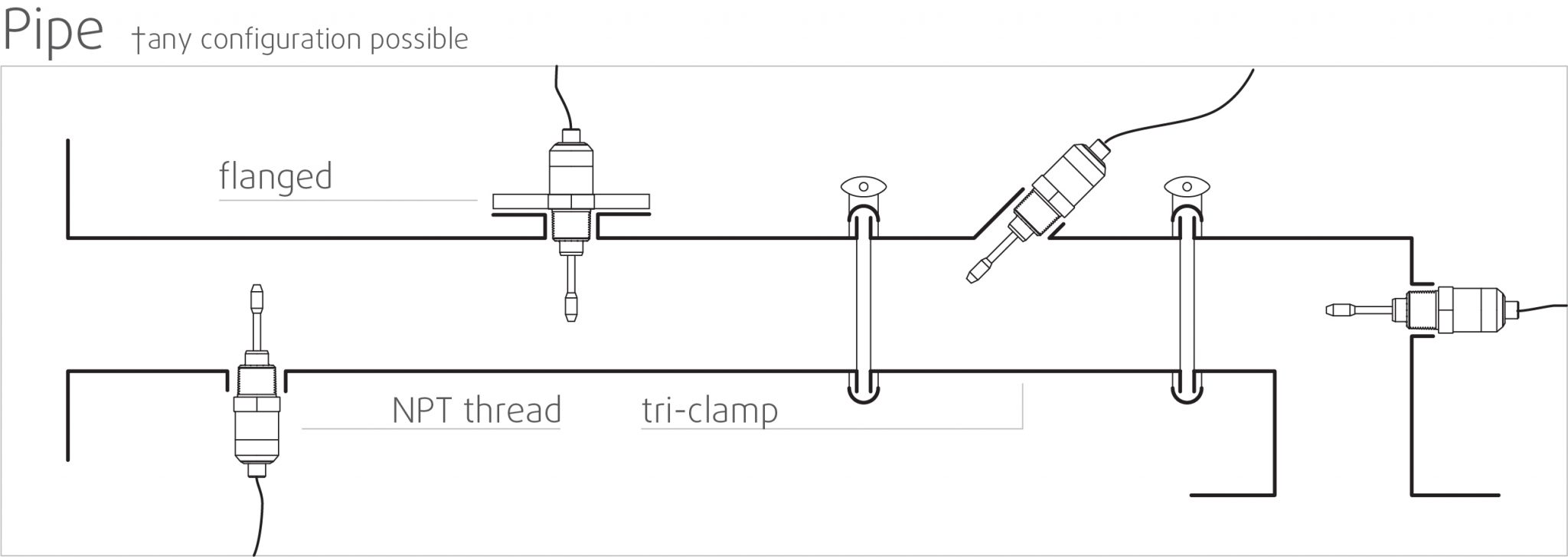
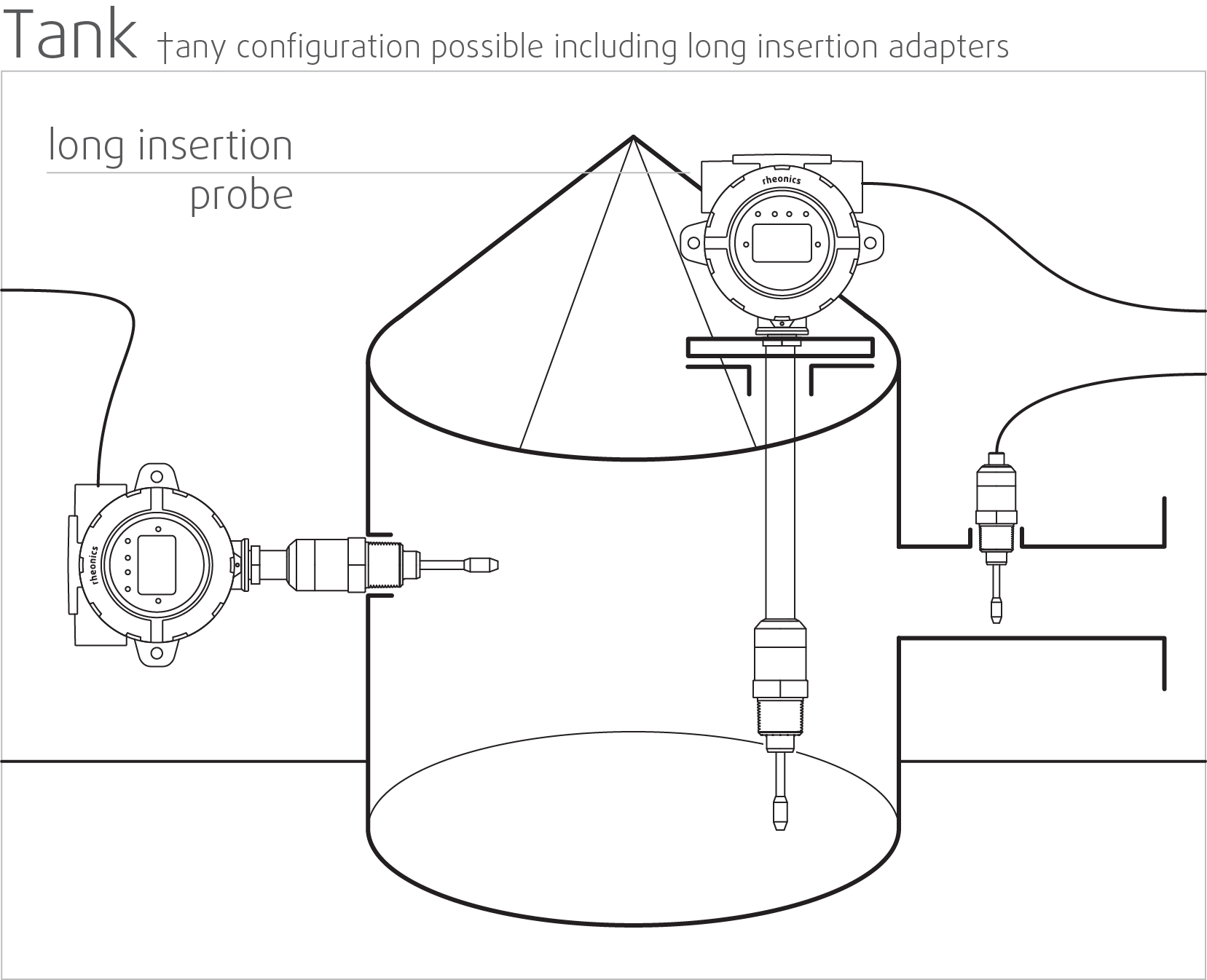
High stability and insensitive to mounting conditions: Any configuration possible
Rheonics SRV and SRD use unique patented co-axial resonator, in which two ends of the sensors twist in opposite directions, cancelling out reaction torques on their mounting and hence making them completely insensitive to mounting conditions and flow rates. Sensor element sits directly in the fluid, with no special housing or protective cage requirements.
Instant accurate readouts on production quality – Complete system overview and predictive control
Rheonics’ RheoPulse software is powerful, intuitive and convenient to use. Real-time process fluid can be monitored on the integrated IPC or an external computer. Multiple sensors spread across the plant are managed from a single dashboard. No effect of pressure pulsation from pumping on sensor operation or measurement accuracy. No effect of vibration.
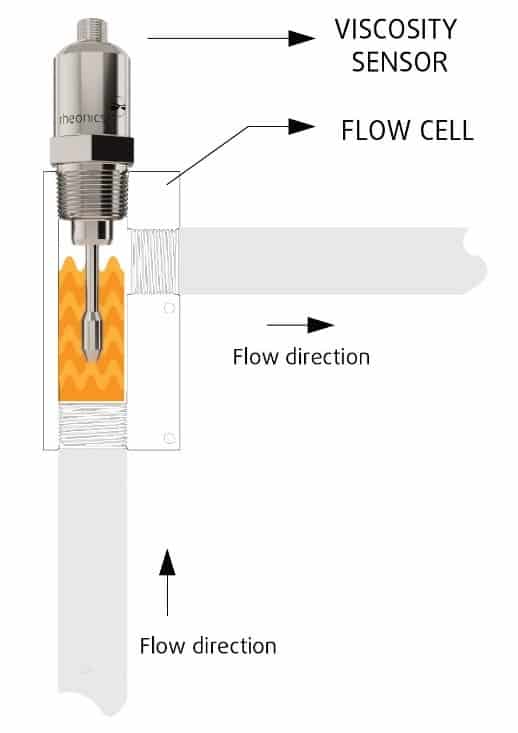
Inline measurements, no bypass line is needed
Directly install the sensor in your process stream to do real time viscosity (and density) measurements. No bypass line is required: the sensor can be immersed in-line; flow rate and vibrations do not affect the measurement stability and accuracy.
ATEX and IECEx Compliance
SRV and SRD are intrinsically safe sensors certified by ATEX and IECEx for use in hazardous environments. These sensors comply with the essential health and safety requirements relating to the design and construction of equipment and protective systems intended for use in potentially explosive atmospheres. The intrinsically safe and explosion proof certifications held by Rheonics also allows for customization of an existing sensor. Custom sensors can be provided for applications that require one unit up to thousands of units; with lead-times of weeks versus months.
Rheonics SRV & SRD are both ATEX and IECEx certified. (Read more)